JPT No. 9 – How to mount an inexpensive sieving lab
Ricardo Araújo1,2, Carlos Natário1, Matthew Pound3,4
1 – Museu da Lourinhã, Rua João de Luís de Moura, 95, 2530-158 Lourinhã, Portugal.
2 – Huffington Department of Earth Sciences, Southern Methodist University, PO Box 750395, Dallas, Texas, 75275-0395, USA.
3 – School of Earth and Environment, University of Leeds, Leeds, LS2 9JT, United Kingdom.
4 – British Geological Survey, Keyworth, Nottingham, NG12 5GG, United Kingdom.
ABSTRACT
We present a new sieving technique designed to recover microfossils from mudstones, clays and poorly consolidated sediments. This new technique is designed to be inexpensive, use the minimal amounts of chemicals and recover well preserved microfossils. The inexpensive nature of this methodology makes it suitable for reconnaissance studies, where finances may be limited. Not using large amounts of chemicals helps to protect the fossils and the environment. Investigations on the Jurassic Lourinhã Formation, Portugal yielded a diverse microfossil assemblage including; archosaur teeth, lizard jaws, amphibian jaws, fish remains, ostracods and charcoal. Such a diverse fossil recovery shows the technique is suitable for painstaking palaeoecological studies as well as reconnaissance work.
INTRODUCTION
The use of confocal laser scanning microscopy (CLSM) for the study of inclusions in amber is a recent development. Böker & Brocksch (2002) produced a series of images of insects in Baltic amber using CLSM and identified the potential for 3D imaging of minute detail of taxonomically important morphological structures such as the mandibles and genital organs. Since then, however, very little has been published on CLSM analysis of amber inclusions despite the apparent benefits.
Sieving methods for microvertebrate recovery have rarely (Mateus et al. 1997) been applied to the Lourinhã Formation. The Lourinhã Formation is comprised of fossiliferous fluviodeltaic deposits that outcrop extensively in the Lusitanian Basin, Western Portugal. This formation is mainly composed of intercalated sandstone channels with extensive alluvial mudstone layers (Hill 1989). Within the extensive mudstone layers most fossils including many large vertebrates (Antunes and Mateus, 2003) and microfossils are found (e.g. Ramalho 1967). Microvertebrate fossil faunas have been collected for many years in the Lourinhã Formation, mainly by surface collecting. However, in 2008 the Museum of Lourinhã started a systematic sieving campaign to better understand the overall fauna of the Lourinhã Formation.
Some of the first microvertebrate finds in Portugal were discovered in the Guimarota mine in 1960 by palaeontologists from the Freilicht Universitat, Berlin (Krebs, 2000). The first account of sieving methods being used in Portugal were published by Kühne (1968), using a constant flow of water and sieves incorportated on a metal barrel. Sieving was applied at the Paimogo theropod embryo nest site (Mateus et al. 1997) from 1994 to 1996, in the search for embryo bones and eggshells (Mateus, 1998) and was also occasionally applied to the Porto das Barcas fossil site, but without much success.
Precise geographical information of sieving sites in the Lourinhã Formation was acquired using GPS coordinates, stratigraphic information and a measured section was acquired by plotting the site photograph with detailed geologic annotation. Samples were taken using a pick axe at 30 – 40cm stratigraphic intervals and within 1 – 2m horizontal spacing. This systematic sieving campaign, applied to the Lourinhã Formation for the very first time, is being used to investigate and assess the composition and diversity of the microfossil fauna.
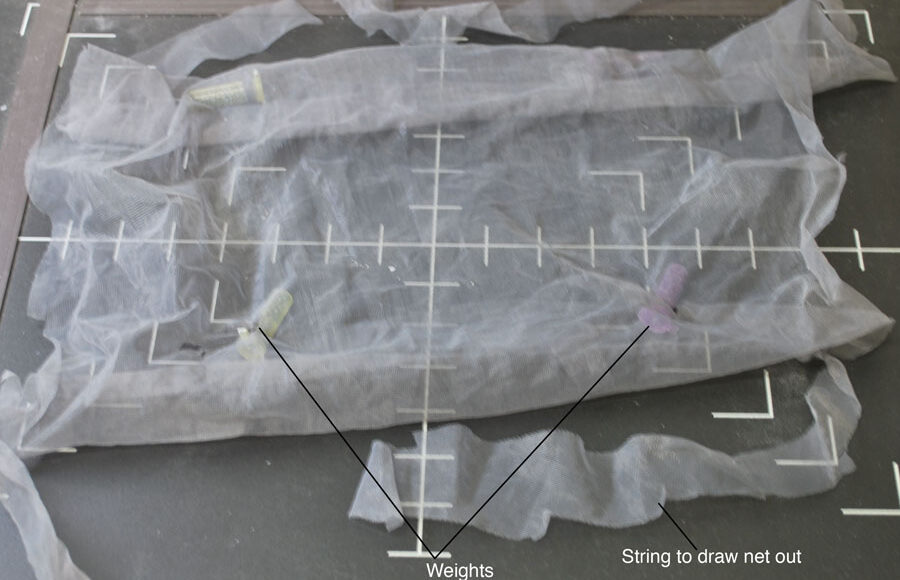
The first use of sieving in the search of microvertebrate remains was performed ca. 1847 by Plieninger, in Germany (McKenna et al. 1994). Early methods of sieving were also used by Moore in England (1867) and later by Wortman and Brown in the United States (1891). Sieving became well implemented in the palaeontological community after Hibbard (1949) reported using the technique to collect Cenozoic mammal fossils from an unconsolidated sandstone matrix (McKenna et al., 1994). Hibbard introduced the use of screen boxes (wooden boxes with a brass mesh). However, this method requires having water near the work site, is laborious, and requires a large staff (Ward 1984). McKenna (1962; 1965) provided further insight into the screen box technique and proposed a standard model using manufactured rectangular wooden boxes and a regular size steel mesh. Both McKenna’s and Hibbard’s techniques are field-oriented and require the presence of nearby water. McKenna simply optimized Hibbard’s technique by processing a larger quantity of matrix using more screen boxes (almost 300, compared to Hibbard’s dozen), and adapting the method to the constraints of particular sites. Grady (1979) described a new method using mosquito nets instead of the classical material of screen boxes with brass mesh, providing a more field-oriented method with easily transported equipment (Ward, 1984).
In contrast, the method reported in this paper is similar to other laboratory-oriented techniques. Described in further detail by Kühne (1971) and Krebs (2000), the “Henkel technique” (Henkel, 1966) is a laboratory-oriented technique that was used for more than a decade during the time that microvertebrates were being collected from the Guimarota Mine. This technique is a static sieve method making use of a jet of water that passes through a barrel with a 500µm -mesh on the side. Freudenthal (1976) developed a table technique. This “table” stood 1m high and used a 500µm mesh as a replacement for the table top. Solid side bars were attached in order to avoid sediment loss. A jet of water was used to wash the sediment through the table top.
The methods above described provide excellent guidance for new field researchers, but due to peculiarities of each site/investigation, modifications to the methods may be required. Aspects such as the location of the fossiliferous horizon (i.e. remoteness), budget and laboratory conditions (i.e. pre-existing infrastructures) can hinder recovery of specimens in an identifiable condition. The methodology here described does not aim to process vast amounts of sediment, as others can. Instead, it does allow individual horizons to be processed without the potential of mixing sedimentary beds. When a sedimentary bed is of limited vertical extent the field-orientated techniques require extensive excavation of the target horizon, losing possible valuable horizons, or mixing multiple sedimentary layers. This can hinder the investigation of fine scale ecological, climatological and evolutionary patterns.